GRANIX was approved through the traditional FDA approval pathway for biologics
Highlights of 351(a) Biologics Licensing Application (BLA) pathway process1
-
Demonstrate safety and efficacy for intended use
-
Demonstrate that the product, manufacturing process, and manufacturing facilities meet applicable requirements to ensure the continued safety, purity, and potency of the product
-
Assessments must also consider the storage and testing of cell substrates and a potency assay
-
Data evidence: pre-clinical and clinical (human) studies
CLINICAL TRIALS
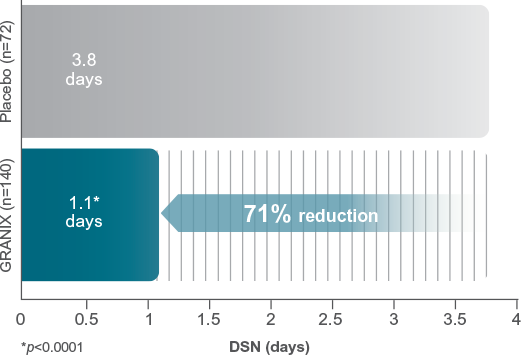
GRANIX demonstrated a significant 71% reduction in duration of severe neutropenia* (DSN) vs placebo2

STUDY DESIGN
The efficacy of GRANIX was evaluated in a multinational, multicenter, randomized, controlled Phase III study of chemotherapy-naïve patients with high-risk stage II, stage III, or stage IV breast cancer receiving a myelosuppressive regimen of doxorubicin (60 mg/m² IV bolus) and docetaxel (75 mg/m²). Comparisons with placebo occurred in the first cycle.2
*Severe neutropenia=ANC <0.5 x 109/L.3
-
GRANIX significantly reduced DSN when compared to placebo (1.1 days vs 3.8 days, p<0.0001)2
GRANIX was approved through the traditional FDA approval pathway for biologics
Highlights of 351(a) Biologics Licensing Application (BLA) pathway process1
-
Demonstrate safety and efficacy for intended use
-
Demonstrate that the product, manufacturing process, and manufacturing facilities meet applicable requirements to ensure the continued safety, purity, and potency of the product
-
Assessments must also consider the storage and testing of cell substrates and a potency assay
-
Data evidence: pre-clinical and clinical (human) studies
CLINICAL TRIALS
GRANIX demonstrated a significant 71% reduction in duration of severe neutropenia* (DSN) vs placebo2
STUDY DESIGN
The efficacy of GRANIX was evaluated in a multinational, multicenter, randomized, controlled Phase III study of chemotherapy-naïve patients with high-risk stage II, stage III, or stage IV breast cancer receiving a myelosuppressive regimen of doxorubicin (60 mg/m² IV bolus) and docetaxel (75 mg/m²). Comparisons with placebo occurred in the first cycle.2
*Severe neutropenia=ANC <0.5 x 109/L.3
-
GRANIX significantly reduced DSN when compared to placebo (1.1 days vs 3.8 days, p<0.0001)2
SAFETY
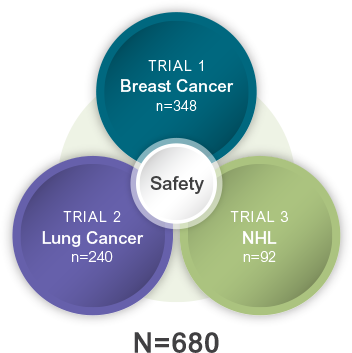
GRANIX safety was analyzed in 3 Phase III studies2

The safety of GRANIX was evaluated in 680 patients receiving chemotherapy for either breast cancer, lung cancer, or non-Hodgkin lymphoma (NHL)2
-
Once-daily dosing2
Both GRANIX and a non–US-approved filgrastim product were administered at 5 mcg/kg subcutaneously once daily beginning 1 day after chemotherapy for at least 5 days
-
Most common adverse reactions2
Bone pain was the most frequent treatment-emergent adverse reaction. The overall incidence of bone pain in Cycle 1 of treatment was 3.4% (3.4% GRANIX, 1.4% placebo, 7.5% non–US-approved filgrastim product)
-
Potential serious adverse events2
The following may occur: fatal splenic rupture, acute respiratory distress syndrome (ARDS), serious allergic reactions, sickle cell disease, glomerulonephritis, capillary leak syndrome, potential for tumor growth, stimulatory effects on malignant cells, leukocytosis, simultaneous use with chemotherapy and radiation therapy not recommended, aortitis
References: 1. Huntington's disease information page. National Institute of Neurological Disorders and Stroke. Accessed January 27, 2022. https://www.ninds.nih.gov/Disorders/All-Disorders/Huntingtons-Disease-Information-Page 2. Nance M, Paulsen JS, Rosenblatt A, Wheelock V. A Physician’s Guide to the Management of Huntington’s Disease. 3rd ed. New York, NY: Huntington’s Disease Society of America; 2011. Accessed January 27, 2022. http://hdsa.org/wp-content/uploads/2015/03/PhysiciansGuide_3rd-Edition.pdf 3. Is Huntington's disease more common than we thought? Press release. American Academy of Neurology. June 22, 2016. Accessed January 28, 2022. https://www.aan.com/PressRoom/Home/PressRelease/1475 4. Walker FO. Huntington's disease. Lancet. 2007;369(9557):218-228. 5. Huntington disease. Genetic and Rare Diseases Information Center – an NCATS Program. Accessed January 28, 2022. https://rarediseases.info.nih.gov/diseases/6677/huntington-disease 6. Tarapata K, Lovecky D, eds. Nutrition and Huntington's Disease: A Guide for Families. New York, NY: Huntington's Disease Society of America; 2010. Accessed January 28, 2022. https://hdsa.org/wp-content/uploads/2015/04/Nutrition-and-HD.pdf 7. Huntington's disease. Mayo Clinic. April 14, 2020. Accessed January 28, 2022. https://www.mayoclinic.org/diseases-conditions/huntingtons-disease/diagnosis-treatment/drc-20356122 8. Simpson JA, Lovecky D, Kogan J, Vetter LA, Yohrling GJ. Survey of the Huntington’s disease patient and caregiver community reveals most impactful symptoms and treatment needs. J Huntingtons Dis. 2016;5(4):395-403. 9. Burgess JC, Davis B, Fogarty E, et al. Caregiver Guide for Mid to Late Stage Huntington's Disease: For Long-Term Care Facilities and In-Home Care Agencies. New York, NY: Huntington's Disease Society of America; 2014. Accessed January 28, 2022. http://hdsa.org/wp-content/uploads/2015/04/CaregiverGuide_Mid_Late_StageHD.pdf 10. Stages of HD progression. UC San Diego Department of Neurosciences. Accessed February 15, 2022. https://neurosciences.ucsd.edu/centers-programs/huntingtons-disease/education/stages-of-progression.html 11. Learn about Huntington's disease: Stages of HD. HD Reach. Accessed February 15, 2022. https://www.hdreach.org/archive/learn/stages.html